12+ Which Intervals Are Affected By The Addition Of A Catalyst
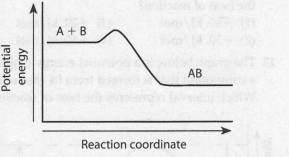
Given the potential energy diagram for a chemical. Web What does adding a catalyst do to equilibrium.

Implement Agile Practices That Work Info Tech Research Group
Bincrease the energy of the products.

. By definition a catalyst is a substance that speeds up a reaction by lowering the activation energy Ea of a chemical reaction. Web The addition of a catalyst during a chemical reaction alters which of the following quantities. Web Regents Chemistry Exam Explanations January 2016 Mr.
This works by lowering the activation energy needed to start the. Meanwhile the concept of catalysis was first researched by chemist Elizabeth. 7 _ÿcŒOšzS 3 1 and 3 4 3 and 4 1 1 and 2 2 2 and 5.
The effect of adding a catalyst to a reaction is to. Web 2CO g O 2 g 2CO 2 g energy. The minimum amount of energy needed for colliding particles to react is called the.
A Entropy B Internal energy C Enthalpy D Activation energy Medium NEET. It increases the rate of reaction. Web 13 Questions Show answers.
Catalysts typically speed up a. Web Catalyst is a term derived from Greek καταλύειν meaning to annul or to unite or to pick up. Web When a catalyst is added to a chemical reaction what will be its effect on the energy of the activated complex and on the rate of the reaction.
This site contains information for AP Chemistry Regents Chemistry and Applied Chemistry. A catalyst lowers the activation energy for example by providing a surface for the reaction. How does a catalyst work in speeding up a reaction.
Web Welcome to CK-12 Foundation CK-12 Foundation FlexBooks 20 has arrived. Save teachers time and engage students with a new simpler interface. Web Which intervals are affected by the addition of a catalyst.
On the potential energy diagram below draw a dashed line to show how the potential energy diagram changes when the reaction is. Web May 26 2014 Rate of reaction can be increased by using a catalyst. Adding a catalyst will increase the rate of reaction significantly.
Web A catalyst is a substance that can be added to a reaction to increase the reaction rate without getting consumed in the process. Web A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. 2NO g O2 g2NO2 g 2NO2 g.
Aincrease the number of collisions between reactants. The energy of the. Web When nitrogen monoxide gas is added to the system the reaction speeds up significantly because it proceeds through the following steps.
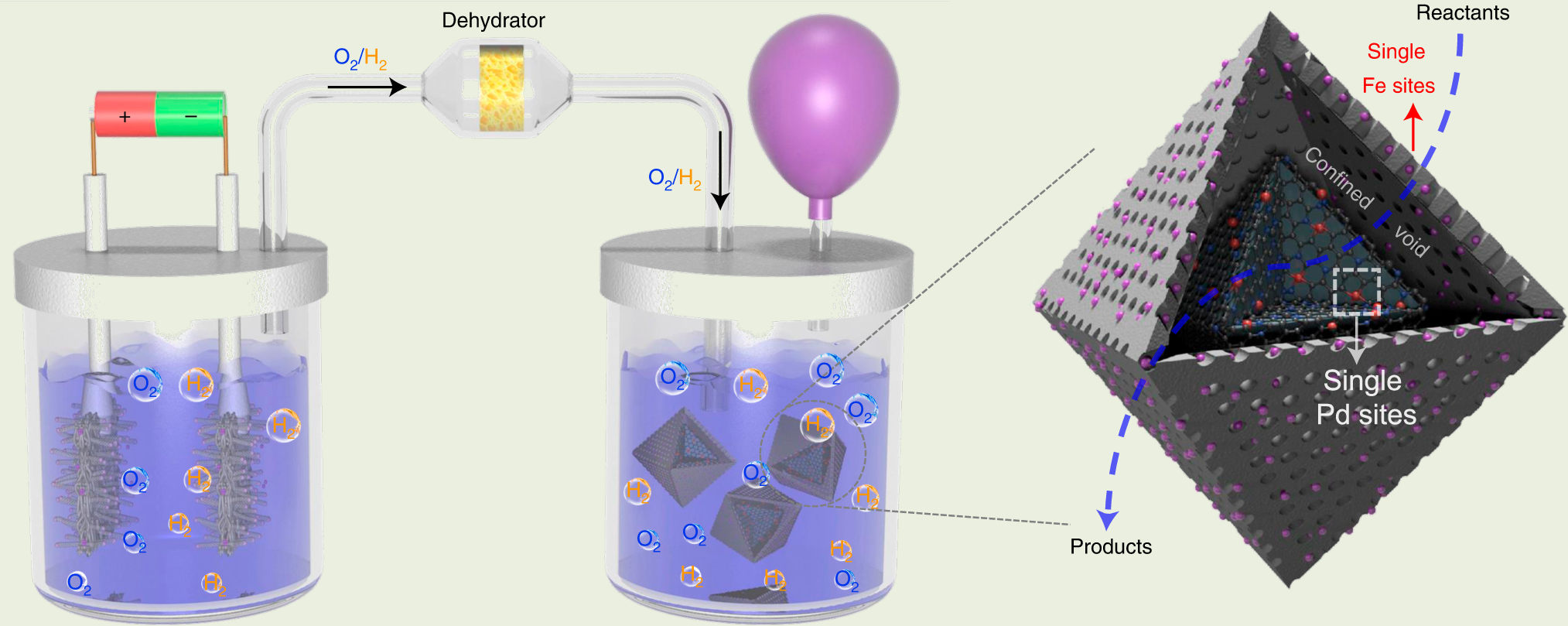
Simultaneous Oxidative And Reductive Reactions In One System By Atomic Design Nature Catalysis
Blog Energy Fitness
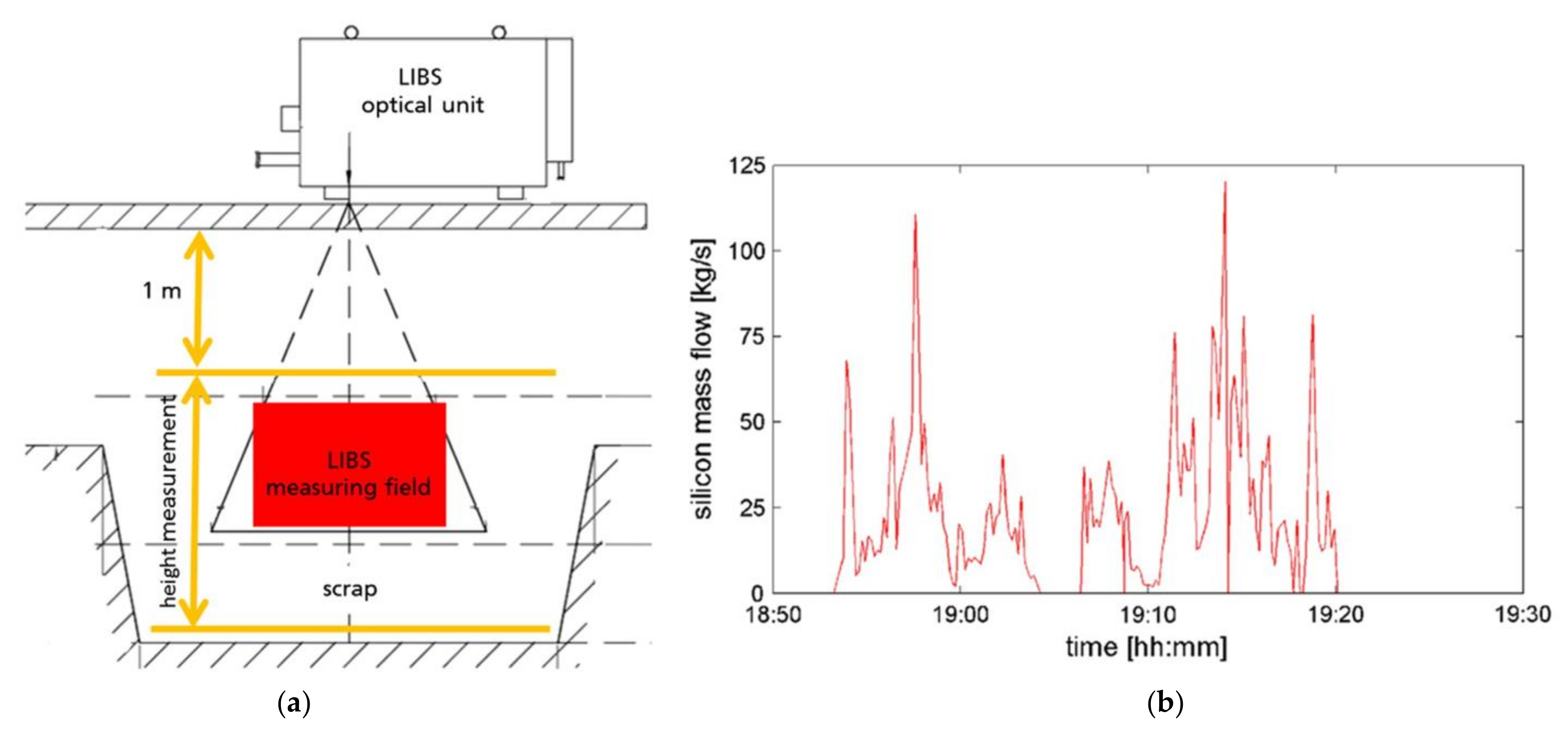
Applied Sciences Free Full Text Review Of Element Analysis Of Industrial Materials By In Line Laser Induced Breakdown Spectroscopy Libs

Seniors Falls In Canada Second Report Canada Ca
Heat Of Reaction Quiz Questions Answers For Quizzes And Tests Quizizz

Mortality Projections For Social Security Programs In Canada

Us4711968a Process For The Hydrofomylation Of Sulfur Containing Thermally Cracked Petroleum Residua Google Patents

Highly Active Urea Functionalized Zr Iv Uio 67 Metal Organic Framework As Hydrogen Bonding Heterogeneous Catalyst For Friedel Crafts Alkylation Inorganic Chemistry

Heterometallic Cobalt Ii Calix 6 And 8 Arenes Synthesis Structure And Electrochemical Activity Rsc Advances Rsc Publishing Doi 10 1039 D2ra01009g

Maintenance Fluconazole Therapy For Recurrent Vulvovaginal Candidiasis Nejm

What Is The Effect Of A Catalyst On The Rate Of A Reaction A Plus Topper

Pecop Blog Read Physiology Educators Perspectives On Educational Issues Including Education Transformation Developing And Using Core Concepts And Competencies Using Evidence Based Innovations In Student Centered Learning Aligning Teaching And

Us4711968a Process For The Hydrofomylation Of Sulfur Containing Thermally Cracked Petroleum Residua Google Patents

Ai59pqhbk1u3vm

Us4711968a Process For The Hydrofomylation Of Sulfur Containing Thermally Cracked Petroleum Residua Google Patents

Pdf Chem F3 Q Ans Full Amour Rashid Academia Edu
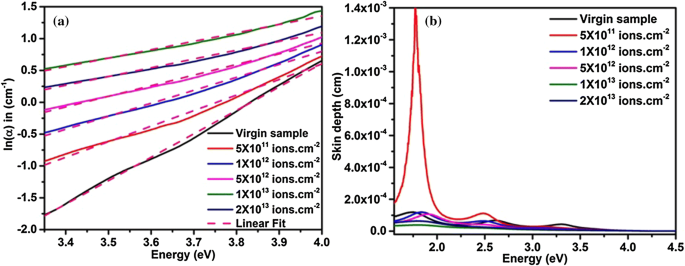
High Energy 120 Mev Au9 Ion Beam Induced Modifications And Evaluation Of Craters In Surface Morphology Of Sno2 And Tio2 Nanocomposite Thin Films Springerlink